Indicolite Tourmaline
Emerald
Neodymium Magnet
Floatation Over Water
Alnico Magnet
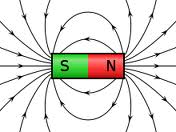
Magnetic fields are generated around the north and south poles of a magnet.
Periodic Table of Elements
"Opposite" fields attract (north to south). "Like" fields repel (north against north, south against south).
1 >
Why Are Gems Magnetic?
In this website, we use the term "magnetic" generically to mean "paramagnetic", referencing the type of magnetism exhibited by most gems that show visible attraction to a magnet. Magnetic susceptibility is the scientific term for the degree to which any material is attracted to or repelled by a magnetic field. Nearly all references to magnetism found in gemological literature tell us that very few gemstones are magnetic, but this is outdated information based on testing with traditional magnets that are relatively weak. With the aid of strong neodymium magnets in conjunction with the floatation method, we have discovered that a large percentage of gemstones show a measurable degree of magnetic attraction.
Magnetic gems are attracted to a magnet because of the metals dissolved within them. The degree of attraction can be noted as weak, moderate, strong, drags or picks up. Many gems are not "magnetic", meaning they are not attracted to a magnet, and these show an inert (diamagnetic) response. We can separate one type of gem from another, and sometimes identify a gem, simply by observing which response a gem shows when the magnetic field of an N52 grade neodymium magnet is applied.
2) Friction, which causes resistance to movement of a gem when the gem is attracted to a magnet, is mostly eliminated. There are a couple of ways to reduce friction: the pendulum method, and the floatation method. The old pendulum method is a less sensitive method because it is hampered by the gravitational weight of the gem, and it is also more cumbersome to use. We use only the simplest and most effective method: floatation of the gem on a raft in water (see page 2 of How to Use a Magnet for details).
These metal oxides can act as coloring agents (chromophores) that determine the body color of a gem. For example, the classic green color of Emeralds is primarily due to the presence of chromium and/or vanadium oxides. The blue color of Indicolite Tourmaline is due to iron ions within iron oxides. Color is the result of light interacting with electrons within a substance. As light rays pass through the crystal structure of a gem, metal ions absorb energy from various portions of the light spectrum, removing specific wavelengths and leaving others that we then perceive as color. This selective absorption of wavelengths gives rise to a large variety of colors and shades.
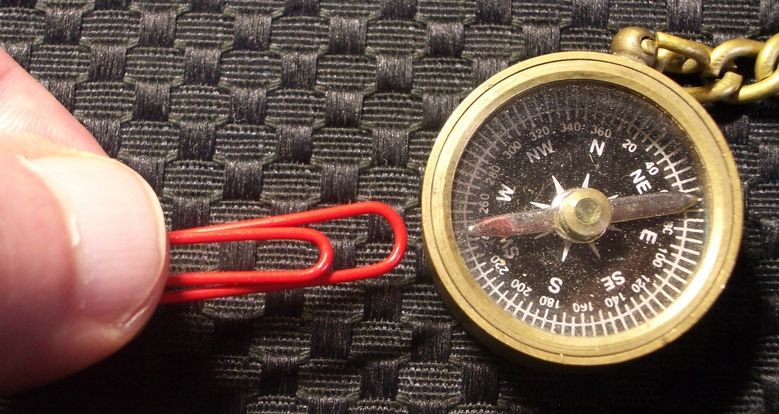
Metal objects made from iron or iron compounds are noticeably attracted to a magnet. A familiar example is a paper clip that sticks to an office magnet. The strong attraction shown by the paper clip is an example of Ferromagnetism ("ferro" refers to iron). The office magnet and paper clip are both composed of ferromagnetic materials, and if we rub a paper clip across the surface of the magnet, the clip becomes magnetized. It retains some of the magnetic field induced by the magnet. The induced magnetic field of the paper clip may then be strong enough to move a compass needle. This simple experiment is an interesting demonstration of ferromagnetism.
Ferromagnetism
There are a number of paramagnetic metals found in nature. In some cases, these metals can exhibit either ferromagnetic or paramagnetic behavior, depending on the state of the metal. For example, when iron exists as a solid pure metal or within a metal alloy, as it does in a paper clip or an iron meteorite, it is ferromagnetic. But when iron exists in the form of charged atomic particles called ions that are dispersed in solid solution throughout the chemistry of a gemstone, it is generally paramagnetic. Each metal ion is a single atom containing unpaired electrons, and collectively these metal ions are responsible for the magnetic attraction we find in many gemstones. In most cases, these metal ions are chemically bonded to oxygen atoms, existing as metal oxides within gems.
Magnetized Paper Clip
Powerful neodymium magnets (neodymium-iron-boron) that can be used effectively to test gemstones have only been available since the 1980's. Neodymium is the rare earth element component, and the magnet surface is plated with nickel.
Overview of Magnetism in Gemstones
Not All Magnets Are Created Equal
Ferromagnetic particles of iron can be detected with a magnet in some synthetic Diamonds, but synthetic Diamonds are the only transparent gemstones we know of that show a visible ferromagnetic response.
Paramagnetism
The magnetism that we most often encounter in transparent gemstones (natural or man-made gems) is a different kind of magnetism called Paramagnetism. This type of magnetism is due to the presence of metals dissolved within the chemistry of the gems. Paramagnetism is a weak type of attraction that can be as much as a million times weaker than Ferromagnetism. Only a temporary magnetic field is involved in Paramagnetism, induced in gems only while the external magnetic field of a magnet is applied. Paramagnetic gems cannot be permanently magnetized. Unlike a metal paper clip, they cannot retain a magnetic field after a magnet is removed from the vicinity.
The combination of a powerful magnet and a near-frictionless testing method provides us with a means to detect very slight magnetism in gemstones. The gem will move either toward or away from the magnet. The magnet we use is an N52 grade neodymium magnet (neodymium-iron-boron magnet, or NIB), which is the strongest grade permanent magnet available today. Weaker grade neodymium magnets such as N42 grade magnets, and other rare-earth magnets such as Samarium-Cobalt magnets, are available, but these are less effective tools because they generate weaker responses. All responses described in this website and listed on the Magnetic Susceptibility Index are based on testing with 1/2" diameter N52 magnets.
People are generally surprised to learn that gemstones can be magnetic. This is because most gems show no direct response to common magnets that we keep around the house. Alnico magnets (aluminum-nickel-cobalt), such as the horseshoe magnet pictured below (left), are many times weaker than rare earth magnets. Ferrite magnets such as the refrigerator magnets pictured below (right), can be even weaker.
Contents of this Section
P.1) Not All Magnets are Cerated Equal
Why Are Gems Magnetic?
P.3) Factors That Affect Magnetism in Gems
P.4) The Magnetic Metals that Color Gems
P.5) Spectroscopy in Relation to MagnetismP.6) Fluorescence in Relation to MagnetismP.7) Quantitative Measurements of Magnetism
Transition Metals
This 3.3ct. Ruby from Thailand is Paramagnetic
Magnetic testing of gemstones as described in this website is only made possible for two reasons:
1) Recent advancements have made small and powerful neodymium magnets commercially available and affordable.
© Kirk Feral 2009-present, All Rights Reserved. These materials may be duplicated for educational purposes only. No part of this website may be duplicated or distributed for profit, for commercial purposes, or for posting to another website without the expressed written consent of the copyright holder.
Among synthetic gems, other Rare Earth elements such as erbium (Er), holmium (Ho), and europium (Eu) are sometimes used to produce pink colors. In the coming pages we'll discover how various metals produce different levels of paramagnetism that result in different magnetic responses in gems.
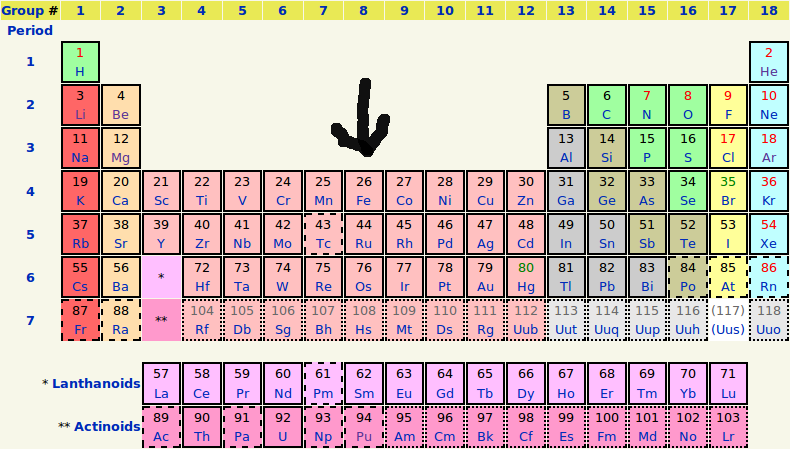
Rare Earth metals occasionally appear in gemstones as paramagnetic coloring agents. There are 17 Rare Earth elements on the Periodic Table (next to the bottom row), but only 3 act as coloring agents in natural gemstones: neodymium, praseodymium and cerium. Uranium is a radioactive paramagnetic metal found as a coloring agent primarily in natural Zircon, and as an activator of green fluorescence in Opal and Chalcedony gems (see the bottom row of the Periodic Table).
A magnetic wand made with an N-52 neodymium magnet can in many cases detect the presence of these metals in gemstones in concentrations that would normally be undetectable without the aid of more complex instrumentation such as a spectrometer.
How Sensitive is Our Magnetic Wand?
Our magnetic wand is quite sensitive. The minimum level of magnetic detectability for different metals is not known due to an absence of formal research, but we can make some rough estimates based on our own research.
Transition metals, Rare Earth metals and Uranium exist within the chemistry of gemstones as oxides such as iron oxide, manganese oxide, chromium oxide and neodymium oxide. A comprehensive list of the molar magnetic susceptibilities for these oxides has been published by CRC Press. Based on the known concentrations of these metal oxides within different gem types that are minimally detectable with our magnetic wand, and based on the known magnetic susceptibilities of these specific metal oxides relative to each other, we came up with some estimates.
Using the Floatation Method, our N52 magnetic wand is able to detect the following paramagnetic metal oxides by weight within gemstones in concentrations as low as approximately:
0.1% iron II oxide (FeO)
0.2% iron III oxide (Fe2O3)
0.13% manganese II oxide (MnO)
0.05% manganese III oxide (Mn2O3)
0.4% chromium oxide (Cr2O3) or vanadium oxide (V2O3)
3.0% copper oxide (CuO)
1.1% nickel oxide (NiO)
0.1% cobalt oxide (Co3O4)
0.07% neodymium oxide (Nd2O3) or praseodymium oxide (Pr2O3)
27.7% cerium oxide (Ce02)
0.3% uranium oxide (UO2)
0.01% erbium oxide (Er2O3)
When paramagnetic metals are present in natural gems in trace amounts (under 0.1%), they are undetectable with a 1/2" X 1/2" N52 neodymium magnet, except for manganese III (Mn3+), neodymium and praseodymium. More exact data on the magnetically detectable concentrations of specific metal oxides within gemstones and the measured magnetic susceptibilities of those oxide concentrations could be determined through research that compares magnetic susceptibility data for specific gem samples to chemical composition data on the same samples obtained through electron microprobe analysis or laser ablation (LA-ICP-MS) analysis. This line of inquiry has not yet been been pursued by professional researchers.
It is the Transition metals, and at times Rare Earth metals, and in rare cases Uranium, that cause color and magnetism in gems. Transition Metals, Rare Earth metals and Uranium occupy a central location in the Periodic Table of Elements. There are 38 Transition Elements, but only 8 play a principal role as coloring agents in gemstones (see the Periodic Table below, elements # 22-29). Transition metals are those metals that transition between different valence states, and most of these transition metals are paramagnetic within gemstones.
Magnetism arises fundamentally from the spin and orbital motion of electrons. When atoms within a metal contain electrons that are not paired with other electrons, the unpaired electrons are free to align themselves with a magnetic field, resulting in magnetic attraction. Some metals such as gold, silver and lead don't have enough unpaired electrons to show magnetic attraction. The same applies to most non-metals and organic substances.
However, every physical object is affected by a magnetic field. All substances in our environment from jellybeans to gemstones respond to the magnetic field of a permanent magnet, either being attracted or repelled by the field. Most of these responses (attraction or repulsion) are too weak to be directly visible when we apply a relatively weak common magnet such as a horseshoe magnet. Common magnets are mostly limited to detecting strong magnetic attraction, as we see with Ferromagnetism, but Ferromagnetic attraction as a rule is not found in gemstones. In contrast, strong neodymium magnets in conjunction with floatation provide a definitive means to view and measure the weak Paramagnetic attraction shown by gemstones, and also to observe weak Diamagnetic repulsion.
Ferromagnetism
Permanent magnets such as neodymium magnets, as well as household and industrial magnets, are manufactured from ferromagnetic materials that are magnetized by a strong electric current. The magnets are then able to generate a strong and persistent ferromagnetic field of their own.
This Gem is Picked Up by a Magnet
Ferrite Magnets
Magnetism in Gemstones
An Effective Tool and method for Gem Identification
© Kirk Feral