Magnetism in GemstonesAn Effective Tool and Method for Gem Identification
Two Sub-groups of Garnets
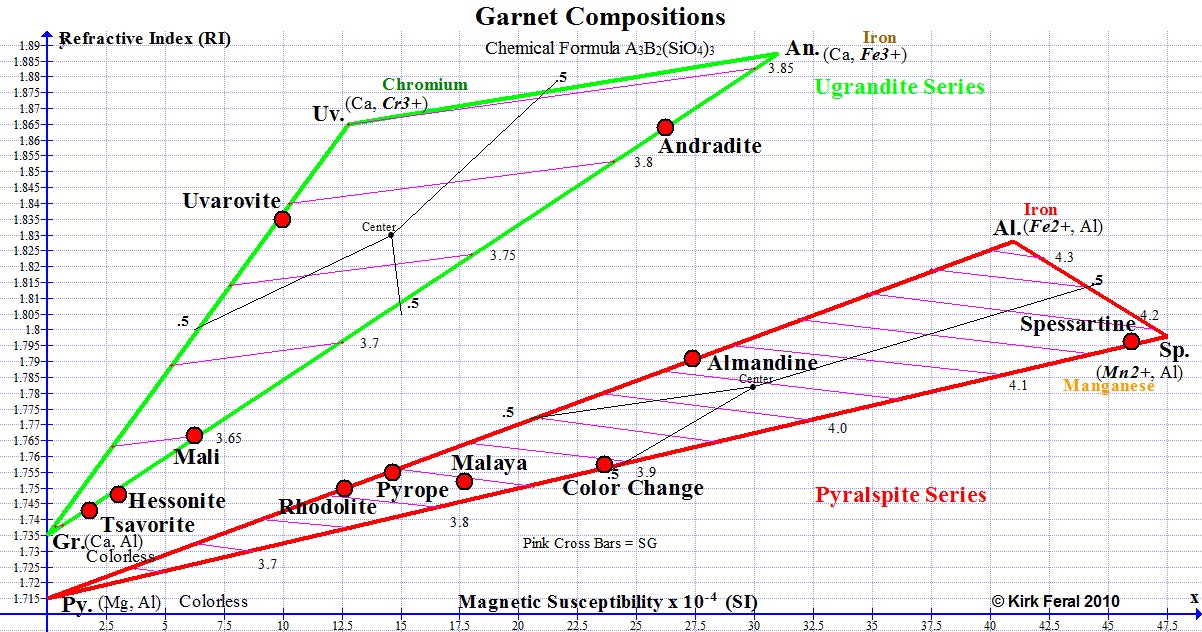
The horizontal axis of the above RIMS graph shows Magnetic Susceptibility increasing from zero all the way to 47.5 X 10 (-4) SI, the highest level of magnetism found in gem Garnets. The further a graph point is to the right, the more magnetic the Garnet. Because Garnets can be 100 times more magnetic than allochromatic transparent gemstones, the notation for Garnet has been changed from 10(-6), which is used on the Magnetic Susceptibility Index for Gemstones, to 10(-4) on our Garnet graphs. To convert, just move the decimal point over 2 places to the right. For example, 12.5 SI for Rhodolite on the Garnet graph above would read as 1250 SI on our Magnetic Susceptibility Index.
The vertical axis shows refractive index increasing from RI 1.70, the lower limit for gem Garnets, all the way to RI 1.89, which is near the upper limit of refractive index known for gem Garnet. The higher the graph point, the higher the RI of the Garnet.
The graph shows two ternaries (triangles), the upper one outlined in green and the lower in red. The green ternary represents Ugrandites, and the red ternary represents Pyralspites. Both series are described below. Gemologists separate the 6 gem Garnet species of the Garnet group into these two sub-groups or series. The 3 species within each ternary intermix with each other in what is termed "solid solution series".
Ugrandite is an abbreviation for the Uvarovite-Grossular-Andradite solid solution series. The most familiar Ugrandites are green due to the presence of chromium and/or vanadium. On the graph, a 2-letter abbreviation for each species name is located at each of the 3 apexes of each ternary, representing the pure state or end-member state of the species. The end-member abbreviation for Grossular is Gr., Andradite is An. and Uvarovite is Uv. Most Ugrandite gems are in solid solution between Grossular and Andradite.
Pyralspite is an abbreviation for the Pyrope-Almandine-Spessartine series. The most common Pyralspites are red primarily due to the presence of ferrous iron (Fe2+), but chromium can also contribute to the red color. The red Pyralspite ternary shows the abbreviations of the 3 end-member species names at the 3 apexes as Al. (Almandine), Sp.(Spessartine) and Py.(Pyrope).
RIMS Graph of Garnet Compositions
Drafted by this author based on the Hoover Diagram
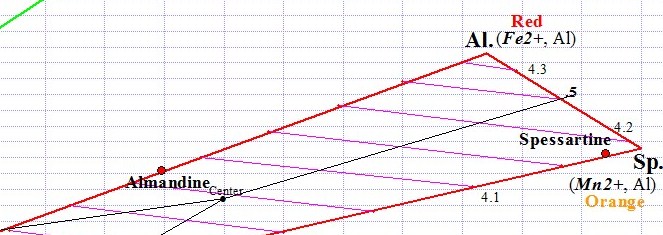
Almandine Trisection
Almandine Trisection
The RIMS diagram (Refractive Index, Magnetic Susceptibility) presented below is a graph showing typical compositions for Garnets of gem quality. It was drafted by the author (Kirk Feral) to serve as a teaching aid for students, and was constructed using a free graphing software program called Graph. The red graph points represent approximate averages for common Garnet species & varieties based on actual measurements of gem Garnets taken by the author. A more comprehensive Graph of All Gem Garnets is presented on page 5 of this section. The graph below provides a handy visual representation of how various types of Garnets are related. Below we’ll examine this graph section by section in order to gain a better understanding of gem Garnets
Specific Gravity and Refractive Index
Pink stripes across each ternary represent specific gravity in 0.1 increments. The specific gravity of any Garnet gem can be estimated from the location of its plot point relative to those pink cross bars. For example, the specific gravity of the Almandine point shown on the graph above can be estimated at 4.07.
Conversely, we can use the graph to estimate the refractive index of a Garnet based on its specific gravity if the SG measurement is precise. This can be useful for OTL gems like Andradites and red Spessartines whose RI's cannot be measured with a standard refractometer.
Specific gravity represents the density of a gem. The denser the Garnet within a species, the higher the concentration of metallic coloring agents within the idiochromatic Garnet, and the higher the magnetic susceptibility.
Refractive index also increases as density increases. This is true not only of Garnets, but of all gemstone species. You can see on the Graph of Garnet Compositions above that Garnets with high RI’s, such as Demantoid and Spessartine are more magnetic than low RI Garnets such as Grossular and Pyrope.
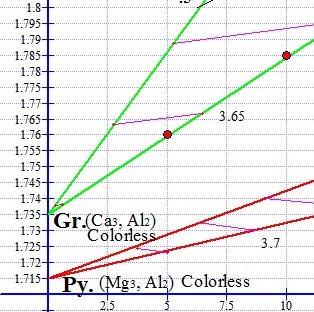
The degree of magnetism increases with the refractive index, and this relationship is directly proportional within each of the two series, Pyralspite and Ugrandite. In the graph shown below left, the red graph point on the lower end of the green ternary represents a green Mali Grossular Garnet oval (pictured below) with a refractive index of 1.76 and a magnetic susceptibility of 5. The upper red graph point on the same green ternary represents a brown Mali Garnet oval (pictured below) with a higher RI 1.785. The brown Mali is exactly twice as magnetic as the green Mali, with a susceptibility of 10.
Magnetism Increases with Refractive Index
Almandine
Mali Garnets
The Hoover diagram is a modification of the Winchell diagram for Garnets, developed by Dr. Horace Winchell in the 1950's as a map for Garnet compositions. Later in 1985, Manson & Stockton published a classic study of Garnets using refractive index, color and spectroscope readings to infer the chemical compositions of individual Garnets. They used an updated Winchell diagram to plot their results.
Dr. D.B. Hoover, along with Bear Williams and colleagues, published a new method for identifying Garnets in 2008 using a further modification of the Winchell diagram that involves magnetism as a variable. Hoover's method permits a much more accurate determination of Garnet chemistry than the Manson/Stockton method because it relies on refractive index and magnetic susceptibility measurements, which are both precise quantitative measurements. In the highly regarded Garnet study by Manson/Stockton, two of the parameters used to approximate Garnet compositions were qualitative assessments: color and absorption spectra using a spectroscope.
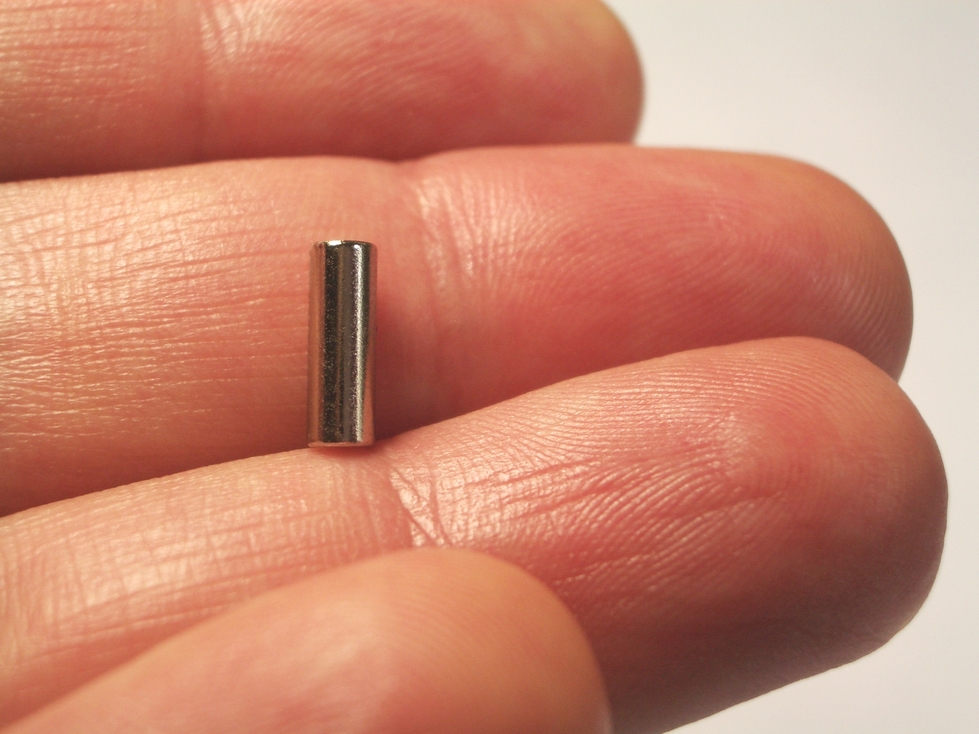
A tiny 1/8" diameter (3mm) neodymium magnet is applied to gems to measure magnetic susceptibility via a Hoover balance.
Notations on the Graph
In the Garnet Composition graph above, parentheses ( ) are around abbreviations for the ions that fill sites A and B, with bold italics highlighting the ion acting as the chromophore. For the Almandine end member, the 2 ions are iron and aluminum, with ferrous iron (Fe2+) as the chromophore. Spessartine has the chromophore manganese (Mn2+), Uvarovite has chromium (Cr3+) and the Andradite end member is colored by ferric iron (Fe3+). Above the parentheses is the predominant color commonly associated with the species, but the colors of 100% pure species are not really known, as Garnet species are never 100% pure. Note that the Pyrope and Grossular end member species can be considered to be allochromatic, with no metallic chromophores represented in their chemical compositions.
Ferrous iron (Fe2+) that colors Almandine results in higher magnetic susceptibility for the Almandine pure end member than does ferric iron (Fe3+) that colors the Andradite pure endmember. We can see on the graph above that the theoretical magnetic susceptibility of pure Almandine is calculated at 41, while pure Andradite is only 31. But gems of these Garnets do not approach the pure end-member state, and we find that the actual ranges of magnetic susceptibility for Almandine and Andradite gems are in reality very similar to each other. Spessartine Garnet colored by manganese (Mn2+) has the highest magnetic susceptibility value (47.5) of all the gem Garnet species. The theoretical pure Pyrope and Grossular endmembers are completely colorless and have magnetic susceptibility values of 0.
Each of the two Garnet ternaries has a central point from which 3 equal sections (trisections) are drawn, one for each of the 3 Garnet species. When a graph point falls within a particular trisection, we conclude that the gem belongs to that particular Garnet species. As you can see below, the Almandine graph point falls within the Almandine trisection. This particular Almandine graph point represents an average of Almandine Garnet readings that we have so far measured. Actual graph points for individual Almandine gems can theoretically fall anywhere within the Almandine trisection, although in reality graph points for Almandine fall fairly close to the line that joins Almandine to Pyrope.
Light green Grossular Garnet is in the Ugrandite series.
Light pink Malaya Garnet is in the Pyralspite series.
© Kirk Feral 2011, All Rights Reserved. These materials may be duplicated for educational purposes only. No part of this website may be duplicated or distributed for profit, for commercial purposes, or for posting to another website without the expressed written consent of the copyright holder.
A Graph of Garnet Compositions
Understanding Garnets through Magnetism
Garnets are the only common transparent gemstones that show a Pick Up response to an N52 magnet. They are more magnetic than other transparent gems because Garnet species are often idiochromatic, with higher concentrations of paramagnetic iron (up to 35% iron oxide by wt.) and/or manganese (up to 40% manganese oxide by wt.). In the case of Uvarovite, the cause of strong paramagnetism is primarily chromium (up to 27% chromium oxide by wt.). High magnetic susceptibility is a key reason we can study Garnets in more detail than is possible with other gemstones. Garnet chemistry can be inferred primarily by determining relative concentrations of iron and manganese.
Hoover Balance
Garnet Magnetism
4.0
© Kirk Feral
Identifying Garnets by their chemical composition is often a destructive, complicated and expensive procedure. Consequently, the chemical composition of a particular gem is not known by most gemologists or students who want to identify a Garnet by species and variety. Usually refractive index, color and absorption spectra are the primary clues to Garnet identification. This is not the case when you have a Hoover magnetic susceptibility balance at your disposal (described on page 4 of Overview of Magnetism).
With a Hoover balance, in combination with a refractometer and Hoover diagram, an individual Garnet gem can be identified by species and variety, and the chemical composition of the gem can also be accurately estimated in terms of the percentages of the gem's 2 or 3 major species components. This method of analysis reveals that Garnet gems sold by dealers are frequently mis-identified as to species and variety.
Almandine
Contains up to 35% Iron
Almandine Trisection
How to Read the Garnet Graph
RI 1.76, SI 5
RI 1.785, SI 10
Spessartine
Contains up to 40% Manganese