Idiochromatic Peridot
(Drags)
Aquamarine:
the light blue gem is weakly magnetic,
the dark blue gem is strongly magnetic
Strong color does not always indicate that a gem contains a high concentration of metal impurities and is strongly magnetic. In some cases, gems that are vibrantly colored by metal ions can be diamagnetic, as shown by the 3 examples below.
Very small concentrations of dispersed metal ions can sometimes be sufficient to create intense colors, as seen in dark blue synthetic Spinels (due to cobalt). Cobalt oxides are strongly paramagnetic, but the gem below (left) shows no attraction to a magnet because the concentration of cobalt is too low to be detectable. In Chrome Tourmaline (center), the lack of attraction is due to low concentrations of the coloring agents chromium and vanadium. In Sphalerite (right), vivid orange color is due to iron involved in a color process called inter-valence charge transfer (see description further below). Gems colored by charge transfer are often diamagnetic due to the low concentrations of the metal ions involved, as is the case with Sphalerite.
Chrome Tourmaline (Inert)
Synthetic Spinel (Inert)
© Kirk Feral 2009-present, All Rights Reserved. These materials may be duplicated for educational purposes only. No part of this website may be duplicated or distributed for profit, for commercial purposes, or for posting to another website without the expressed written consent of the copyright holder.
In Beryl gems, ferrous iron (Fe2+) is responsible for higher magnetic susceptibility than ferric iron (Fe3+). Gem treatments such as heat and irradiation can change the valence states of these metal ions that cause color in gems.
Idiochromatic or Allochromatic: This is closely related to concentration. Attraction to a magnet tends to be greater in idiochromatic gems such as Garnet and Peridot (colored by iron), and gems such as Rhodochrosite and Rhodonite (colored by manganese). Many idiochromatic gems will show a direct response to a magnetic wand without the need for floatation.
Idiochromatic gems are self-colored, meaning color is due to metals that are part of the inherent chemistry of the gem. The chemical formula of a gem species tells us whether or not the gem is idiochromatic. The written formula includes one or more metals, which can cause color and magnetic susceptibility. Any natural transparent gemstone larger than 0.5ct that is magnetic enough to be picked up by a magnet is an idiochromatic gem. Garnet and Peridot are the only idiochromatic minerals that are commonly faceted as gems.
Of all the common or primary transparent gemstones, Spessartine Garnet has the highest magnetic susceptibility, and this is due to a high concentration of manganese. After Garnets, Peridot is the most magnetic transparent primary gemstone, due to iron. One of the most magnetic transparent rare or secondary gemstones that this researcher has tested is Axinite (see our 3-page section on Axinite). Ferroaxinite is an idiochromatic mineral species. Due to high iron content, Ferroxinite gems are nearly as magnetic as Peridot, yet less magnetic than Spessartine and most other Garnets.
Snowflake Obsidian (Drags)
Mahogany Obsidian (Picks Up)
Idiochromatic Axinite
(Drags)
Opaque gemstones, whether allochromatic or idiochromatic, can be exceptionally magnetic. Opaque Obsidian, Jasper and black Tourmaline all show very strong magnetic susceptibility due to high concentrations of iron.
Idiochromatic Spessartine Garnet
(Picks Up)
Allochromatic Indicolite & Verdelite, & Yellow Tourmaline (all Drag)
Concentration of Metals: Magnetism in gemstones is not only determined by the types of metal ions present, but also the concentration of those ions. Magnetic attraction directly corresponds to the concentration of transition metals within a gem, which can only be quantitatively determined by advanced methods of chemical analysis. Colorless and near-colorless gems typically show no magnetic attraction because they lack sufficient metallic chromophores. Gems that are darker in color often have higher concentrations of metal impurities and are more magnetic than lighter stones of the same type. For example, as Chrysoprase color becomes more saturated due to higher nickel content, these gems become more magnetic. Chrysoprase responses can vary widely from inert to strong. Such correlation is also clearly seen in blue Aquamarine Beryl, where increasing intensities of blue color and magnetic response are due to increasing concentrations of iron. Near-colorless Aquamarine can be inert.
Factors that Affect Magnetism
Inter-valence Charge Transfer: This is a color process that can create color and modify color in gemstones that contain transition metals. Color arises when electrons jump from one transition metal to another. For example, in iron to titanium charge transfer (Fe2+-Ti4+), the valences of the two ions change to Fe3+ and Ti3+. Two other examples of inter-valence charge transfer in gems are iron to iron (Fe2+-Fe3+) and manganese to titanium (Mn2+-Ti4+).
Strong color can be produced even when the metals are in very low concentrations. The concentration of iron involved in iron to titanium charge transfer can be a thousand times less than the concentration of dispersed iron ions that would be needed to create the same intensity of color. The concentration iron involved in iron to titanium (Fe2+-Ti4+) charge transfer is too low to induce detectable magnetic susceptibility, and gems colored primarily by this process are often diamagnetic. It's possible that charge transfer between other metal ion pairs such as Fe2+-Fe3+ that influence gem color might affect the magnetic susceptibility of a gem differently than Fe2+-Ti4+ charge transfer, but this is another area of gemology that is yet to be researched.
Unlike gems that are primarily colored by color centers (as described on the previous page titled Diamagnetic Gems), gems colored by intervalence charge transfer can show magnetic attraction due to the presence of additional metal ions that may or may not be involved in producing color. The photos below are examples of 3 gems that derive their colors from iron-titanium (Fe2+-Ti4+) charge transfer: a blue Sapphire from Sri Lanka, an orange Dravite Tourmaline, and an Andalusite. Only the Andalusite shows magnetic attraction (Weak).
Theory vs. Practical Application: Valence Chemistry in gemstones and color processes such as inter-valence charge transfer are aspects of Crystal Field Theory and Ligand Field Theory. These are hugely complex subjects that are often not thoroughly understood even by the scientists who specialize in those areas of research. Fortunately, we can use a magnet to identify gems without knowing anything about the types of metals, valence states or color processes involved in those gems.
Blue Sapphire (Sri Lanka, Inert)
Dravite Tourmaline (Inert)
Let's look a closer look at the effects of valence changes in Beryl. Pictured below are 2 large and beautiful Beryl Gems, one green and the other blue. The color of Green Beryl is a blend of blue color due to ferrous iron (Fe2+) and yellow color due to ferric iron (Fe3+). On average, Green Beryl shows weaker magnetic responses than Aquamarine, since Green Beryl contains less ferrous iron (Fe2+) and more ferric iron (Fe3+). Blue Aquamarine is colored primarily by ferrous iron (Fe2+), and is more magnetic. The magnetic responses of Green Beryl range from Inert to Weak, while Aquamarine responses range from Weak to Moderate.
Heat treatment is used to remove the yellow color in Green Beryl (as well as in blue-green Aquamarine) in order to change the color to a purer blue that is generally considered more desirable for Aquamarine gems. Heat treatment changes gem color by changing the valence state of the iron ions within these gems from Fe3+ to Fe2+. We might expect that this treatment increases the magnetic susceptibility of the gems. Experimentation on individual Beryl gems before and after heat treatment would be needed to verify this assumption, as heat treatment also reduces the overall magnetic susceptibility of a gem, regardless of the valence sate of the metals.
Unheated Green Beryl (Weak) & Heated Aquamarine Beryl (Moderate)
Andalusite (Weak)
Sphalerite (Inert)
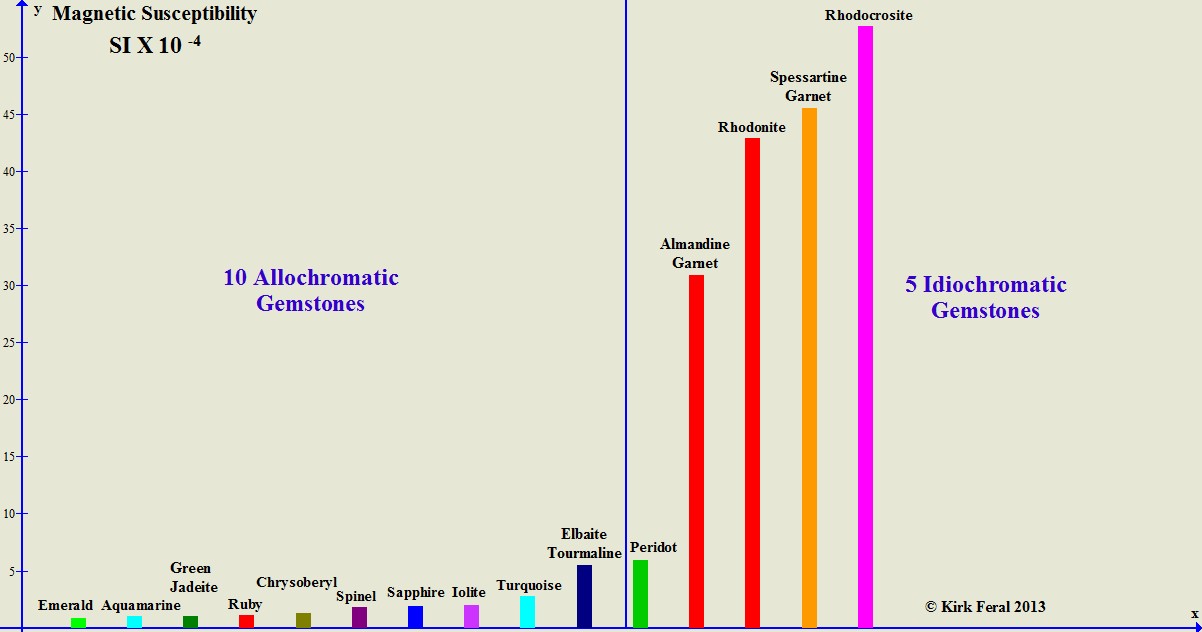
The bar graph below compares the magnetic susceptibility (or "degree of magnetic attraction") of 10 allochromatic gemstones compared to the magnetic susceptibility of 5 idiochromatic gemstones. The gems are ordered from least magnetic to most magnetic. The height of each colored bar represents the maximum magnetic susceptibility we were able to measure for each type of gemstone. The most magnetic idiochromatic gemstone shown on the graph- Rhodochrosite- can be up to 250 times as magnetic as any of the least magnetic allochromatic gemstones that have barely measurable susceptibility.
A Comparison of Magnetic Susceptibilities of Allochromatic vs. Idiochromatic Gems
Higher concentrations of transition metals within a gemstone species not only increases the measured magnetic susceptibility (represented as SI), but also increases refractive index (RI) and raises density (specific gravity, SG). In natural stones, this is most noticeable in idiochromatic gems such as Garnet. For example, the refractive index of Almandine Garnets can increase from RI 1.773 in a gem with magnetic susceptibility of SI 2200 (below left) to a refractive index of RI 1.795 in a gem with a higher magnetic susceptibility of SI 2600 (below right). However, in the case of Almandine Garnet, color intensity does not increase with a higher concentration of iron.
Almandine Garnet (low RI 1.773)
Pink YAG (anomalously high SG 5.7)
Spessartine Garnet (Picks Up)
Tanzanite (Diamagnetic)
Any change in color that results from the heat treatment or irradiation of any gem, whatever the gem species, is always due to changes in the valence states of the metal ions that cause gem color. Changes in magnetic susceptibility due to heat treatment are likely small and may not be noticeable with a magnetic wand. For an example of how heat treatment can affect the valence states and magnetic susceptibilities of Sapphire gems, see our Sapphire & Ruby section.
For information about how the Floatation method can be used to detect variations in magnetic susceptibility, see page 2 of How to Use a Magnet for Gem Identification.
Most Tourmaline gems belong to the Elbaite species, which is considered allochromatic. Due to the relatively high iron content found in blue Indicolite Tourmaline and green Verdelite Tourmaline, these are the most magnetic of all transparent allochromatic gemstones. They show a Drag response to an N52 magnet. Yellow Tourmaline can be equally magnetic due to manganese. Most brown Dravite Tourmalines are not attracted to a magnet due to low iron content. For more details about color and magnetism in Tourmalines, see the section titled Tourmalines.
Peridot is a trade name for the green gem variety of the Forsterite species within the Olivine group. Although Peridot is generally referred to by gemologists as an idiochromatic gem, 90% of its composition is allochromatic Forsterite, which is colorless when pure. Peridot is also partly composed of idiochromatic Fayalite, which is a species of opaque mineral colored brown by iron. Typical green Peridot contains only about 10% Fayalite, which contributes the iron that produces green color and magnetic attraction in Peridot. Peridot (green Forsterite) could be correctly referred to as an allochromatic gem, as are Elbaite Tourmaline, Grossular Garnet and Pyrope Garnet. All of these gems are colorless when pure, with color and magnetic attraction due to mixing with idiochromatic species within their own mineral group. Near-colorless Peridot with just a hint of color does exist. Perhaps it would be most accurate and informative to refer to all of these gems, including Peridot, as a blend of idiochromatic and allochromatic species, with color and magnetism produced by metal ions from the idiochromatic species.
Peridot Composition is Primarily Allochromatic
The vast majority of gemstones are allochromatic. They tend to show weaker magnetic responses than idiochromatic gems because allochromatic gems generally have lower concentrations of metallic chromophores. These coloring agents exist only as impurities that are not essential to the gem's chemistry. Most allochromatic gems must be floated for any magnetic attraction to be visible. When completely pure, all natural allochromatic gems are colorless and diamagnetic.
Blue Sapphires (such as the low-iron Sri Lankan Sapphire pictured above left) that show no magnetic attraction derive all of their blue color from Fe2+-Ti4+ charge transfer, but most blue Sapphires contain additional iron and show some magnetic attraction (see the Sapphire & Ruby section). Dravite Tourmalines colored primarily by iron-titanium charge transfer are diamagnetic. The Andalusite above (right) and the dark blue Sapphire from Madagascar pictured below show weak magnetic attraction due to additional iron ions that are not involved in the iron to titanium charge transfer process. Titanium ions within gems (Ti4+ and Ti3+) cannot by themselves cause color or magnetic susceptibility.
This Dark Blue Sapphire from Madagascar is Weakly Magnetic
Yttrium Aluminum Garnet (YAG) is a synthetic Garnet that is allochromatic, with the rare earth metal erbium added during synthesis to create pink color. The intense pink YAG pictured below has such a high concentration of erbium that its level of magnetic susceptibility reaches SI 5200, higher than magnetic susceptibility of any natural Garnet.
The density we look for to identify man-made YAG gems is about SG 4.5, but the unusually high erbium content in the YAG gem below raises the density to SG 5.7, mimicking the high density of Cubic Zirconia. This is because the density of erbium metal itself is high (SG 9.1). In allochromatic gems, the densities of all transition metal and rare earth metal impurities are higher than the densities of the gems they color, and therefore increasing concentrations of these metals can increase the refractive index and density of the gems.
Almandine Garnet (higher RI 1.795)
Allochromatic vs. Idiochromatic
Effects of valence on magnetic susceptibility can be related to concentration of metals. For example in Tourmaline, manganese in the valence state of Mn3+ causes red color, but only very low concentrations of Mn3+ are needed to create strong color. Red Tourmaline is inert to weakly magnetic. In contrast, manganese as Mn2+ is involved in yellow color in Tourmaline. High concentrations of Mn2+ are often found in yellow Tourmaline, and these Tourmalines are often strongly magnetic.
Red Tourmaline (Mn3+), Weak
Yellow Tourmaline (Mn2+), Drags
Because metallic coloring agents like chromium and vanadium can be magnetically undetectable in gems, it follows that the measured magnetic susceptibility and magnetic response of a gemstone are not necessarily related to the gem's color. Often more than one type of transition metal is present within a single gem. As we have already mentioned, traces of iron impurities may contribute to gem’s magnetic response without contributing to its color. The types of metals that cause color in gems are discussed in detail on the next page.
The magnetic susceptibility of a gem is the measurable degree to which the gem is attracted to or repelled by a magnetic field. The visible response that a gem shows to a magnet generally corresponds to its measured magnetic susceptibility. Since the degree of repulsion (diamagnetism) is consistently very weak among gems, we measure only the degree of attraction with a magnetic susceptibility balance. The degree of magnetic attraction that a gem shows can vary widely and is dependent on the gem's chemical composition, particularly the types of metals and concentration of those metals within the gem.
Types of Metals: Some metals have much stronger magnetic susceptibilities than others. Iron and manganese oxides in gemstones can be strongly magnetic and are often easily detectable. An example is strongly magnetic orange Spessartine Garnet, colored by manganese. Chromium and vanadium oxides have lower magnetic susceptibilities and occur in lower concentrations, and these metals are rarely detectable with a magnet in gemstones. As an example, blue Tanzanite colored by vanadium is diamagnetic.
Almandine Garnet Picks Up Due to High Concentration of Iron
Valence State: Besides the concentration of metal ions and their varying magnetic susceptibilities, the valence state of these ions is also a significant factor that affects the degree of magnetism in gems. Valence also affects color. Valence refers to the gain or loss of electrons by an atom, resulting in an affinity to bond with other atoms. As an example, iron ions within gems can exist in 2 different valence states: ferrous iron (Fe2+) or ferric iron (Fe3+).
Cause of Color: Transition metal ions and rare earth metal ions dispersed throughout a gemstone can create or influence gem color, and these are often the same metals that cause magnetic attraction. But this is certainly not always the case. For example, iron and cobalt both contribute to the color of natural blue Spinel, but only iron is responsible for the magnetic attraction and measurable magnetic susceptibility seen in blue Spinel. The concentration of cobalt in Spinel is generally too low to contribute to magnetic attraction. On the Magnetic Susceptibility Index for Gemstones, we list Cause of Color as a reference, not as the designated cause of magnetic susceptibility for any particular gem variety or species.
Blue Spinel (Moderate)
In some cases, the color of a gem may be caused by chromium, vanadium, copper or cobalt impurities, but magnetic attraction shown by that gem is due entirely to iron or manganese impurities. The iron or manganese may contribute nothing to the color of the gem. Strong colors in gemstones can also be caused by color centers (vacancies in the crystal lattice) or charge transfer processes, and the concentration of transition metal ions that may affect those color processes may be too minuscule to create any magnetic attraction.
Chrysoprase:
the light green gem is inert,
the dark green gem is strongly magnetic
Magnetism in Gemstones
An Effective Tool and Method for Gem Identification
© Kirk Feral