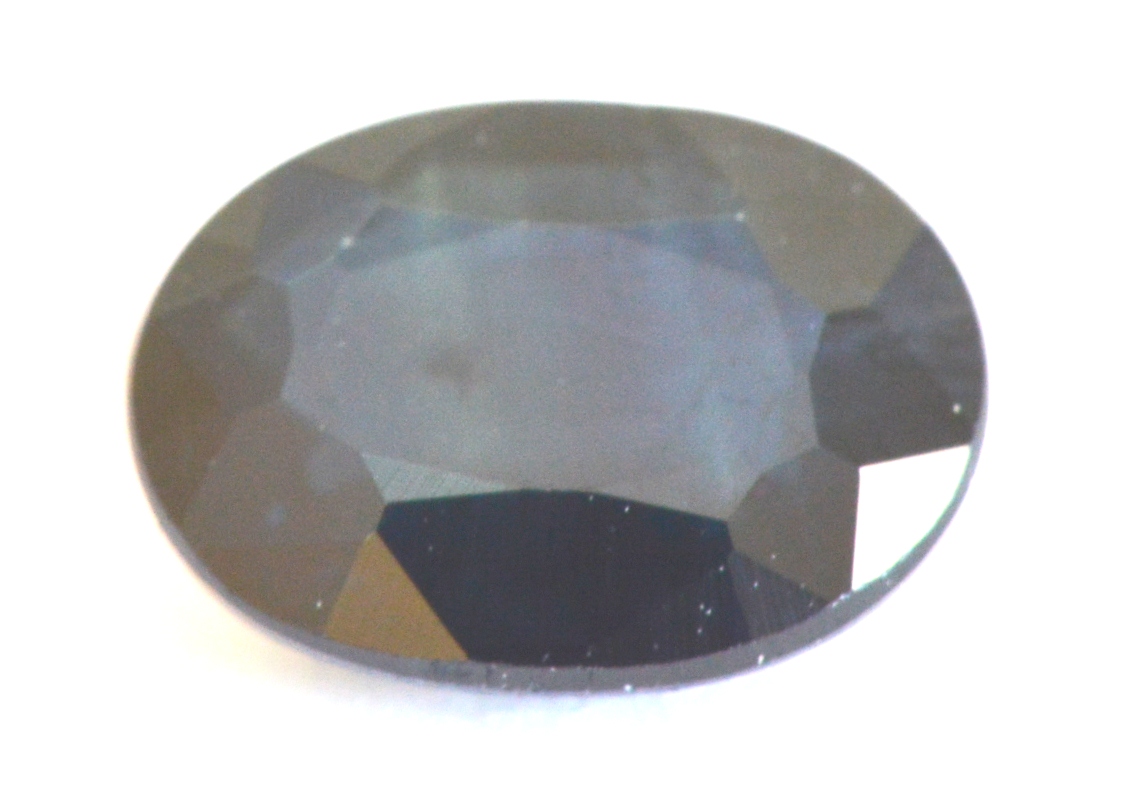
Natural Blue Sapphire,
Sri Lanka, Weak, (SI < 20)
Iron is involved in the coloration of synthetic blue Sapphire as it is in natural blue Sapphire, so how do we explain differences in magnetic response? Through the process of inter-valence charge transfer, ions of iron (Fe2+) and titanium (Ti4+) interact with each other to cause blue color in Sapphire. Fe2+ loses an electron to become Fe3+, and Ti4+ gains the electron to become Ti3+. This process is such a strong cause of color that only a trace amount of iron (too low to be magnetically detectable) is all that is necessary to create vivid blue color through Fe2+-Ti4+ charge transfer.
In most natural blue Sapphires, there is also some "surplus" iron, perhaps mostly Fe3+. This additional iron may exist as cryptic (colorless) iron, or possibly become involved in iron-to-iron (Fe2+-Fe3+) charge transfer, which may modify blue color. The over-dark blue color that is often seen in blue Sapphires with high iron content is due to maximized iron to titanium (Fe2+-Ti4+) charge transfer, and also possibly to Fe2+-Fe3+ charge transfer. Additional iron not involved in Fe2+-Ti4+ charge transfer or Fe2+-Fe3+ charge transfer is probably cryptic and likely responsible for all of the detectable magnetic attraction seen in most natural blue Sapphires. This extra iron can also be detected with a spectrometer, as shown in the graph below. In synthetic Sapphires, there is no detectable "excess" iron present, only a small amount of iron in Fe2+-Ti4+ charge transfer, and therefore we see no attraction to a magnet.
Blue Sapphire
Natural vs. Synthetic: Natural Sapphires that are pink, purple or blue can at times be diamagnetic. Therefore, diamagnetic gems of these colors cannot reliably be distinguished by magnetic response from synthetic Sapphires. However, natural blue Sapphires most often do show a weak to moderate magnetic response due to iron, and such responses definitively separate them from synthetic blue flame fusion Sapphires, which are always inert (diamagnetic). We can say that any magnetic attraction rules out synthetic origin for blue Sapphire, but a diamagnetic response does not rule out natural origin.
Vivid Blue Sapphire
S. Madagascar, Weak (SI 22)
Natural vs. Synthetic: One way to distinguish between natural and synthetic transparent Corundum is to look for inclusions. Unlike natural Corundum, synthetic Corundum (flame fusion type) generally has no inclusions that can be seen with a loupe or gem microscope. Angular growth lines seen under magnification are another indication of natural origin, while curved growth lines indicate synthetic origin.
A magnetic wand can also provide a useful means of distinction. As we have noted, "fancy color" natural Sapphires with green, yellow, orange, and Padparadscha colors are often weakly to moderately magnetic due to iron ions involved in color. Synthetic Sapphires (flame fusion type) of all colors lack sufficient iron to show any attraction to a magnet. They are always diamagnetic, and any magnetic attraction in Sapphire of any color indicates natural origin (with the rare exception of flux-grown synthetic Sapphire).
Natural Padparadscha Sapphire
Brazil, Weak (SI 43)
Synthetic Padparadscha Sapphire
Diamagnetic (Inert)
Blue gems from all these low-iron areas can have vivid “cornflower” violet-blue colors and very low or undetectable magnetic susceptibilities. The rich blue color of these gems is derived primarily from Fe2+-Ti4+ charge transfer rather than from ferrous iron (Fe2+) alone or Fe2+-Fe3+ charge transfer. Examples with darker blue color, higher iron and greater magnetic susceptibility can also be found in these regions. However, a diamagnetic (repel) response during floatation shown by a natural blue Sapphire of medium to dark color points toward an origin of Sri Lanka, Burma, Tanzania or southern Madagascar. A weak response from any dark blue Sapphire also points toward an origin of one of these low-iron regions.
Kashmir Sapphire
Photo taken at the Los Angeles Museum of Natural History
Vivid Blue Sapphire
Burma, Diamagnetic
The most desirable blue colors in Sapphire are associated with low-iron metamorphic Sapphires, which are mined primarily in Sri Lanka, Burma, Tanzania, and southern Madagascar, with an obscure deposit in Baffin Island, Canada. Blue Sapphires from Sri Lanka are often an attractive light blue, and frequently show no magnetic attraction. More than a century ago, vivid blue metamorphic Sapphires were actively mined in the now-depleted mines of Kashmir in the Himalayas, and these Kashmir Sapphires set the "gold standard" for blue color in Sapphire.
Sapphire and Ruby
Since ancient times, Sapphire and Ruby have been two of the most beautiful and disirable gemstones. Ruby and Sapphire are ancient trade names for different color varieites of the mineral species Corundum. Corundum is simply aluminum oxide, and features high brilliance and high hardness. When pure, it is colorless and diamagnetic, composed of only 2 elements: aluminum and oxygen.
Sapphire
Ruby
Inclusions: Star Sapphire gems, as well as star Rubies, are translucent to nearly opaque. Most derive asterism from dense needle-like inclusions of Rutile. Pure Rutile needles are diamagnetic and do not affect the magnetic responses of Corundum. However, in the case of Black Star Sapphire, needles of Hematite are responsible for the asterism. In this instance, the iron content of the Hematite results in a strong magnetic response when floated. Black Star Sapphires are the most magnetic of all gem Sapphires, with susceptibilities as high as SI 143.
Black Star Sapphire
Thailand, Strong, (SI 143)
Iron (Fe3+) and color centers involving Fe3+ are the primary causes of yellow color, and Fe3+ also plays an important role in the color and magnetism of green and brown Sapphire. Vanadium may also play a role in green color. Orange and Padparadscha (orangey pink) colors are primarily due to small magnetically undetectable amounts of chromium (Cr3+ and color centers involving Cr3+), although iron can also contribute color and magnetism in these Sapphires. Most natural "fancy color" Sapphires show some degree of magnetic attraction, but in some cases iron content is too low to be magnetically detectable.
We found a number of natural yellow Sapphires to be diamagnetic. Just a few parts per million of iron (Fe3+) in color centers are sufficient to produce strong yellow color. However, high concentrations of dispersed iron (Fe3+) in Sapphires can also be associated with yellow color and magnetic attraction, as shown below right. Brown and orange Sapphires also can be diamagnetic, and responses range from inert to moderately magnetic depending on iron concentration.
Regardless of geographic origin, green Sapphires on average have higher magnetic susceptibilities than the other colors of transparent Sapphires that we tested due to relatively high concentrations of iron in the form of Fe3+. We have not encountered any diamagnetic green Sapphires, but magnetic responses still only range from Weak to Moderate (SI 69-135). The green, brown and yellow Sapphires pictured below are all moderately magnetic, with the dark green gem showing the highest magnetic susceptibility of any transparent Sapphire in our study. For comparison, the most magnetic blue Sapphire tested is a dark blue gem from northern Madagascar with a susceptibility of only SI 99. The only Sapphires we found to be strongly magnetic are opaque black Sapphires.
Brown Sapphire
Thailand, Moderate (SI 130)
Blue Sapphires from other locations most often have geologic origins that are basaltic (igneous, volcanic) rather than metamorphic. These higher-iron basaltic gems often show darker “ink” blue colors with gray or black undertones due to significant Fe2+-Fe3+ charge transfer in addition to Fe2+-Ti4+ charge transfer. Dark blue Sapphires from these regions tend to have significantly higher total iron content (up to ten times the concentration found in low-iron Sapphires) and consequently show greater magnetic susceptibility. Responses to a magnetic wand are often Moderate rather than Inert or Weak.
Such gems are found in Australia, Thailand, Cambodia, Viet Nam, Laos, China, Nigeria, Rwanda, Mozambique, northern Madagascar, Colombia, Brazil and Montana. (For more detail on Sapphire origins, see the article "Inside Sapphires" by C.P. Smith). Of course, pale blue and vivid blue gems with lower iron content can also be found in any of these regions. However, as long as the gems are not extremely pale, all blue Sapphires from these areas will likely contain sufficient iron to show some attraction to a neodymium magnet.
Dark Blue Sapphire
Australia, Moderate (SI 61)
Dark Blue Sapphire
Northern Madagascar, Moderate (SI 99)
Treatments: Although it has not yet been directly studied, high heat treatment of natural Sapphire of any color to enhance color and clarity can theoretically lead to a change in magnetic susceptibility as the valence state of the iron ions is changed from Fe3+ to Fe2+. Comparisons of heated vs. unheated blue Sapphires suggest that heat treatment reduces the overall magnetic susceptibility of Sapphires. In keeping with increased levels of divalent iron (Fe2+), heat treatment also seems to significantly hamper long wave UV fluorescence.
As Fe3+ iron in blue Sapphire transforms to Fe2+ iron with high heat, the amount of charge transfer apparently increases between Fe2+ iron and titanium (Ti4+), and Fe2+-Fe3+ charge transfer may decrease, resulting in brighter and more intense blue color. We can also speculate that the dissolution of Rutile needles (titanium oxide) within blue Sapphire during heat treatment may also result in more Ti4+ ions becoming available for charge transfer with Fe2+.
The total amount of Fe2+ iron directly involved in Fe2+-Ti4+ charge transfer remains small and magnetically undetectable. Pictured below, the light blue unheated Sapphire on the left is weakly magnetic while the darker blue heated Sapphire on the right is diamagnetic, indicating that magnetic susceptibility is not necessarily linked to intensity of color. Research on individual Sapphires before and after heat treatment is needed to understand the effects of heat on magnetism, and the degree to which changes in magnetic susceptibility due to heat can be detected with a magnet.
Unheated Blue Sapphire
Southern Madagascar, Weak (SI 39)
Heated Blue Sapphire
Sri Lanka, Diamagnetic
Yellow Sapphire
Sri Lanka, Moderate (SI 117)
Fancy Color Sapphire
Color Variations: Natural Sapphires are found in a variety of colors other than blue, and these are often referred to as "fancy" Sapphires. Pink Sapphire is colored primarily by chromium, as is Ruby, but the concentration of chromium is lower in pink Sapphire than in Ruby. The small amount of chromium in pink Sapphire is not magnetically detectable. Purple Sapphire is colored by chromium along with iron-to-titanium charge transfer (pink + blue = purple). Recent studies (Dubinsky, et al., 2020) have shown that trace amounts of vanadium can also contribute to purple color. Color Change Sapphire changes from purple to pink depending on the light source. Color Change Sapphire may contain trace amounts of vanadium in addition to chromium, iron and titanium. When iron content relative to chromium increases, pink Sapphire can change to purple, and magnetic susceptibility can increase.
Pink and purple are the least magnetic of all Sapphire colors, with magnetic responses ranging from Inert to Weak. Examples of Sapphires that show Diamagnetic responses (no magnetic attraction) have been encountered among these 2 colors, as well as among some blue Sapphires. As we would expect, colorless Sapphires also show no magnetic attraction.
As we look at magnetism in Corundum, we will focus on the primary causes of color. Corundum's magnetic susceptibility is generally low. In our study of over 60 Sapphires and 50 Rubies, we found that both Sapphires and Rubies show magnetic responses that range from Inert (Diamagnetic) to Moderate, with low magnetic susceptibilities ranging from < 0 (less than zero) to 135 SI X 10(-6). In comparison, Spessartine Garnet is on average 100 times more magnetic than Sapphire and Ruby.
Purple Sapphire
Sri Lanka, Weak (SI 56)
Pink Sapphire
Madagascar, Diamagnetic
Green Sapphire
Unknown Origin, Moderate (SI 135)
© Kirk Feral 2014, All Rights Reserved. These materials may be duplicated for educational purposes only. No part of this website may be duplicated or distributed for profit, for commercial purposes, or for posting to another website, without the expressed written consent of the copyright holder.
Macro-inclusions that are magnetic are rarely encountered in transparent Sapphire gems. The anomalous green Sapphire pictured below has unusually high magnetic susceptibility (SI 191) due to black platelet inclusions in the lower right portion of the gem. A pinpoint wand reveals that the unusually strong magnetic response is due to the inclusions. We can speculate that the inclusions may be Hematite.
Yellowish Green Sapphire with Magnetic Inclusions
Sri Lanka, Strong, (SI 191)
White Star Sapphire
Sri Lanka, Diamagnetic
Natural Color Change Sapphire
Tanzania, Weak (SI 40)
Synthetic Color Change Sapphire
Diamagnetic (Inert)
Synthetic Blue Sapphire
Diamagnetic (no attraction)
Country of Origin: In our study, we encountered diamagnetic blue Sapphires from Sri Lanka, Burma, Tanzania and Baffin Island, Canada. These all share similar conditions of geologic formation (metamorphic compression within a host rock of marble, schist or gneiss) that create low-iron Sapphire. The magnetic responses of blue Sapphires can therefore give us clues about their geographic origins.
Concentration of Iron: Among natural untreated Sapphires that are mined at the same geographic origin, gems generally show increased color intensity as the concentration of iron increases. This correlates with increased magnetic susceptibility from lighter to darker stones, as demonstrated by the two Sapphires pictured below, both unheated and both from Sri Lanka. The light blue gem is diamagnetic, and the dark blue gem is weakly magnetic. In addition to total iron content, charge transfer processes involving iron also drive color intensity. Because the iron content within a blue Sapphire can at times be very low, we occasionally run into natural blue Sapphires that, like synthetic blue Sapphires, show no attraction to a magnet.
This Light Blue Sri Lankan Sapphire is Diamagnetic &
This Dark Sri Lankan Blue Sapphire is Weak (SI < 20)
Natural Sapphire Contains Surplus Iron
Vivid Blue Sapphire
Sri Lanka, Diamagnetic
Fluorescence: Sapphires of any color can fluoresce under long wave UV light if iron content is low enough that it doesn’t quench fluorescence. Fluorescence colors include pink and red (due to chromium) and orange (due to F-centers or crystal lattice defects according to Vigier & Fritsch, 2023). Strong pink or red fluorescence in a blue Sapphire is a good indication that the gem is natural, while synthetic flame-fusion blue Sapphires are mostly inert or show only weak pink or weak red fluorescence due to low chromium content. Flux-grown synthetic blue sapphires that I've tested fluoresce yellowish-green.
Most natural blue Sapphires that fluoresce strongly are unheated, lighter in color and diamagnetic, but there is no consistent correlation between fluorescence, color intensity, and magnetic susceptibility. Some diamagnetic unheated Sapphires with light blue color show no fluorescence, while some moderately magnetic heated Sapphires with dark blue color can fluoresce strongly due to higher chromium content. Iron quenches fluorescence, but the other key determinant of fluorescence in Sapphire is the concentration of chromium (which is not magnetically detectable in Sapphire).
Chromium is found in relatively high concentrations in pink, purple and Padparadshca (pinkish orange) Sapphires, and these are the colors that fluoresce pink and red most consistently and intensely. But chromium can also cause pink/red fluorescence in natural Sapphire when the concentration of chromium is too low to create body color or significantly modify body color. We find that blue, brown and even colorless Sapphire can fluoresce pink/red due to the presence of chromium. Natural green Sapphires in our study show little or no fluorescence due to high iron and low chromium content. Yellow Sapphires fluoresce orange, which might be due to F-centers, or to a combination of pink fluorescence from chromium and yellow fluorescence from another activator.
Colorless Sapphire, Diamagnetic
Daylight & UV Fluorescence
Blue Sapphire, Weak, SI <20
Daylight & UV Fluorescence
Page 1 2 Next >
1 >
Sapphire
Cobalt glass is now being used to fill cracks in low-grade colorless and blue Sapphire, creating composite gems with vibrant blue color out of stones that would otherwise not be of gem quality. The cobalt glass can cause red fluorescence. Cobalt is also used in surface diffusion of blue Sapphire. It is unlikely that the amount of cobalt used in either glass-filling or diffusion treatment contributes to detectable magnetic susceptibility in Sapphire.
Cobalt Glass-filled
Sapphire
Cobalt-Diffused
Blue Sapphire
Light Blue Sapphire
Sri Lanka, Diamagnetic
The Sapphires used for this study come from diverse geographic origins, and include both heated and unheated gems. Nearly all were transparent faceted gems. No rough specimens or opaque stones were part of this study, as these may conceal magnetic inclusions. Also, only one flux-grown synthetic Sapphire and one flux synthetic Ruby were tested, as these synthetics may contain magnetic flux particles of Platinum.
Flux-grown synthetic Sapphires are rare compared to flame fusion synthetics, and they have different identifying characteristics. Blue flux-grown sapphires are grown over many months into hexagonal crystals that have a somewhat natural appearance. A flux-grown synthetic blue sapphire may contain residual metallic flux inclusions that cause magnetic attraction, mimicking the response of natural blue sapphire.
Flux-grown sapphire can also show unequal color distribution that resembles natural color zoning. Fortunately, the synthetic nature of flux-grown blue sapphire is readily revealed under longwave UV light if yellow-green fluorescence is observed.
In "Golden Sheen" Sapphires, needle inclusions of ilmenite (iron-titanium oxide) along with Hematite create a yellowish-brown reflective sheen. This reflectivity causes the gems to appear opaque, even though the corundum is actually transparent in transmitted light. Golden Sheen Sapphires that we’ve tested are moderately to strongly magnetic when floated, with magnetic susceptibility ranging from 86 to 139. We can assume the susceptibility is due to iron within the needle inclusions.
Golden Sheen Sapphire
Moderate (SI 86)
Magnetism in Gemstones
An Effective Tool and Method for Gem Identification
© Kirk Feral
The difference in the two Corundum varieties lies in the metal impurities that color them. Iron (Fe2+ and Fe3+) and titanium (Ti4+) are the key metals involved in the color of blue Sapphire, while chromium (Cr3+) is the primary coloring agent in Ruby. The complete story of color is very complex, involving a number of trace elements, inter-valence charge transfer, color centers and even silicon (J. L. Emmett et al., 2017).