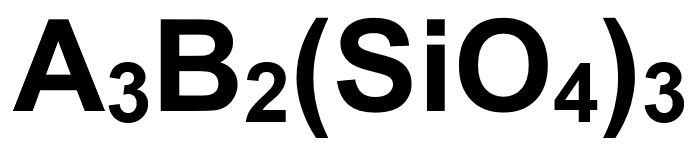Garnets are some of the most magnetic gemstones, and magnetic testing gives us rare insights into the world of Garnets, revealing how the different species and varieties of Garnet are related. For most people, the term Garnet brings to mind a dark red gem, but Garnets are in fact a diverse group of gemstones, with more species and color varieties than any other type of transparent gemstone.
Uvarovite Druse
Synthetic Garnets are manufactured today for industrial use, but they play only a limited role as gemstones. The synthetic Garnet GGG (Gadolinium Gallium Garnet) was once popular as a simulant for Diamond and colored stones, but it is no longer manufactured for gem purposes. The synthetic Garnet YAG (Yttrium Aluminum Garnet) is still created in a number of colors and faceted to imitate a variety of natural gemstones, but YAG gems are sparse in the marketplace compared to other types of synthetics and imitations. Neither of these Garnets are found in nature. See page 14 for a detailed look at synthetic gem Garnets.
There are now over 20 species of natural Garnet recognized by mineralogists, but most are of little interest to gemologists. Only 6 Garnet species produce gem-quality crystals that are fashioned into gemstones. These are Almandine, Pyrope, Spessartine, Grossular, Andradite and Uvarovite. Almandine, a red to purplish red gem, is the most common species. Gem-quality Uvarovite is mainly found in druse form (clusters of tiny green crystals), and Uvarovite jewelry is primarily fashioned from druse slabs rather than individual crystals.
Grossular Garnet Cubic Crystal from Canada
Blue Color Change Garnet
In addition to red, Garnets are found in every color, from colorless to black, pink, purple, blue, green, orange, yellow, and even iridescent. Some Garnets change color from daylight to incandescent light, much like Alexandrite. There are also hundreds of subtle color variations among gem-grade Garnets. Some varieties have only been discovered in the last few decades, and some are among the world's rarest gemstones. This section offers a tour of all of them, and presents a considerable amount of new information about Garnets never before published, achieved through extensive study by this author.
© Kirk Feral 2011, All Rights Reserved. These materials may be duplicated for educational purposes only. No part of this website may be duplicated or distributed for profit, for commercial purposes, or for posting to another website without the expressed written consent of the copyright holder.
The World of Garnets
What are Garnets? The Garnet group falls within the family of Orthosilicate minerals, all with related chemical compositions. Other Orthosilicates include Peridot, Zircon, Topaz, Andalusite, and many secondary or less common gems such as Kyanite and Sphene. What all these gemstones share in common is the chemical component SiO4 (one silicon atom per 4 oxygen atoms) in their chemical formulas.
Garnets belong to the cubic crystal system, and therefore gems are isotropic (singly refractive). However, under a polariscope, anomalous double refraction is common in red and green Garnets due to distortion of the isotropic cubic crystal structure that may can occur during metamorphic conditions of high pressure and high temperature.
Garnets are found all over the world. Some of the most unusual gem varieties come from African countries such as Namibia, Tanzania and Madagascar. Garnets are one of the few primary or common gemstones that are generally not treated, although Demantoid, and occasionally Hessonite (Bear Wiliams, pers. comm., 2011), can be heated to enhance color.
The extreme diversity seen in Garnets is the result of different species mixing together in endless variation, similar to what we see among different species of Tourmaline. We'll see how magnetic testing can reveal exactly how different Garnet species have blended within an individual gem. Based on analysis of over 500 Garnets, we present a new proposed Gem Garnet Classification system, as well as the first Graph of All Gem Garnets showing composition ranges for all known gem Garnets.
Rhodolite Garnet from Burma
Hessonite Garnet from Nigeria
Garnet Magnetism
Garnets are Found in Many Colors
Why So Many Varieties?
Aside from the 6 natural gem Garnet species, gemologists and gem dealers separate Garnets into many different varieties based on variations in color and color phenomena. To learn why there is such tremendous color variation in Garnets, it’s helpful to have a basic understanding of the chemical formula shown below. The letters A and B simply represent sites where ions of specific chemical elements can bond with silicon and oxygen to give a Garnet a chemical composition unique to its particular species.
In Garnet, the A and B sites can be partially filled by calcium, magnesium or aluminum ions, none of which cause color or magnetic attraction. But idiochromatic Garnet species contain magnetic coloring agents that occupy these sites. The four principal paramagnetic metals that can cause color in Garnets by occupying sites A or B are iron (purplish red, brown, yellow), manganese (orange, pink), chromium (red, green), and vanadium (green). Combinations of these chromophores in varying concentrations result in a bewildering variety of color and subtle variations in hue. Charge transfer processes likely also play a role in creating and modifying color in some Garnets, although this is not well understood as yet by researchers. When chromium is present, Garnets with low iron content (Grossular Garnets and Pyrope-Spessartine Garnets) also fluoresce pink or red under long wave UV light.
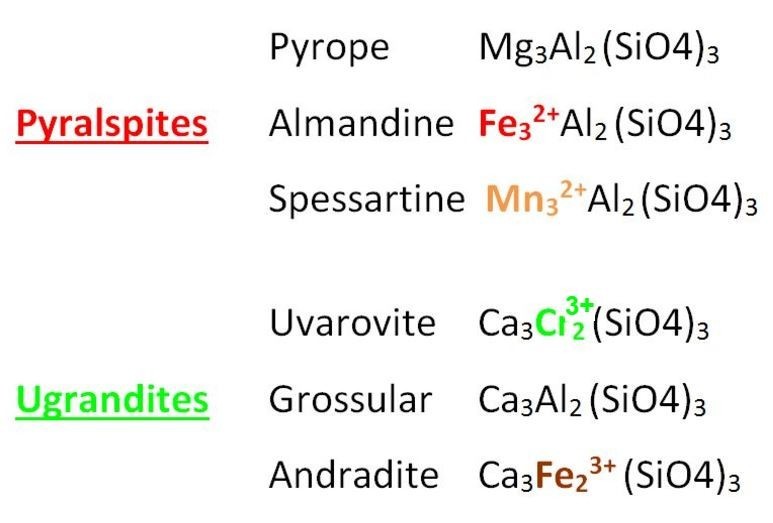
The Chemical formulas for the six species of gem Garnet are shown below. These formulas represent the composition of the pure un-mixed Garnet species, referred to as End Members. The chromophores (color-producing metal ions) are highlighted in color, with the valence number added here in superscript for clarification. Chromophores are found at the A site in Pyralspite Garnet species, and at the B site in Ugrandite Garnet species.
The 100% pure end-member Garnets shown above don't exist in nature. As you can see by its chemical formula, Pyrope is allochromatic, and pure Pyrope Garnet would be colorless, as there are no transition metal chromophores in the A or B sites, only magnesium and aluminum. The graph point for pure Pyrope would plot at zero magnetic susceptibility, indicating it would be diamagnetic. Pure colorless gem Pyrope is not known, but rare near-colorless gems do exist. The most common Pyrope gems are red and significantly magnetic due to mixing with other Garnet species such as Uvarovite/Knorringite (chromium) and Almandine (iron).
Pure Grossular Garnet is also allochromatic and would also be colorless and diamagnetic because there are no chromophores in the A and B sites, only calcium and aluminum. In this case, some Grossular gems do approach the pure endmember in composition, and examples of pure colorless gems do exist. These are called Leuco Garnets, though in most cases these Garnets retain a hint of color and a measurable amount of magnetism. We have not yet encountered a diamagnetic colorless Leuco Garnet.
Near-colorless Grossular Garnets:
Hessonite, Green Grossular, Mali
Contents of this Section
Pg 1) The World of Garnets
Pg 2) Understanding Garnets through Magnetism
Pg 3) How Magnetic Are Garnets?
Pg 4) A Rainbow of Color in GarnetsPg 5) Graph of All Gem Garnets
Pg 6) Almandine, Pyrope, SpessartinePg 7) Color Change GarnetPg 8) Malaya GarnetPg 9) Pastel Pyrope
Pg 10) Rhodolite & Chrome Pyrpe
Pg 11) Ugrandites- Andradite, UvarovitePg 12) Ugrandites- GrossularPg 13) Distinguishing Between Garnet Species and Varieties
1 >
1 >
Orange Spessartine Garnet
Pastel Pyrope Garnets
Almandine
Magnetism in Gemstones
An Effective Tool and Method for Gem Identification
© Kirk Feral