Green: Green and yellowish green Paraiba color is likely due to a combination of blue color from copper (Merkel & Breeding, 2009) and yellow color from manganese (Mn2+ and Mn2+-Ti4+ charge transfer). Iron is present only in trace amounts, but it has been suggested that green color in Paraibas may also be partly due to traces of iron involved in iron-to-titanium (Fe2+-Ti4+) charge transfer (Laurs, et al., 2008). Green Paraibas tend to have higher concentrations of manganese than Paraibas of other colors, and consequently green Paraibas as a whole show the strongest magnetic attraction. The green gems pictured below both show a Drag response, and the gem on the left has the highest measure magnetic susceptibility of any Paraiba Tourmaline tested for this study.
Paraiba Tourmalines
Overview of Paraiba Tourmalines
Elbaite Tourmalines whose colors are primarily influenced by copper impurities are referred to in the trade as Paraiba, Paraiba-type, cuprian or copper-bearing Tourmalines. Copper imparts blue color. Paraibas are rare. They were discovered in the state of Paraiba, Brazil in 1987, but since 2001 they have also been mined in Nigeria and Mozambique. Here we refer to all these copper-bearing Tourmalines as Paraiba Tourmalines, regardless of origin.
Paraiba Tourmaline Rough Crystals
(5.47ct. Total,Weak to Strong)
Color Varieties
Blue: Copper imparts the blue color that is associated with Paraiba Tourmalines. Pure blue Paraibas are colored entirely by copper ions, which exist in concentrations too low to be magnetically detectable. Color intensities range from pale blue to deep blue. Pure blue may be modified to greenish blue or "aqua" blue by the presence of manganese (Mn2+). The presence of Mn3+ can sometimes lead to pink or red color zoning within the blue body. Blue gems show magnetic responses that range from inert to strong depending on the concentration of manganese (Mn2+). Blue Paraiba gems that are inert (diamagnetic, SI <0) have an extremely low and magnetically undetectable concentration of manganese. An Inert response by any blue Tourmaline can be considered diagnostic for Paraiba Tourmaline colored entirely by copper, as opposed to blue Tourmaline colored by iron. The strongly magnetic gem pictured below owes all of its blue color to copper and and all of its strong magnetism to manganese (Mn2+).
Magnetic Susceptibilities of Paraiba Tourmaline Color Varieties
The graph below shows the measured magnetic susceptibilities of 25 copper-bearing Tourmalines tested in this study. Each column of dots represents a color variety: blue, purple & violet, and green. Each individual colored dot represents a single gem.
Blue Tourmalines colored by iron and iron-iron charge transfer can look identical to blue Paraiba Tourmalines colored by copper (see photos below). We have examined numerous examples of Tourmalines from Brazil and other sources that were suspected to be copper-bearing due to their unusual blue "Paraiba" color, but spectrographic analysis proved that they were colored by iron rather than copper. We found that the magnetic susceptibility range of blue copper-bearing Tourmalines (due to manganese) largely overlaps the magnetic susceptibility of blue Tourmalines colored by iron (due to iron), but a diamagnetic response was found only in stones colored by copper. Blue Paraiba Tourmalines in this study showed responses ranging from Inert (Diamagnetic, SI <0) to Strong (SI 273), with magnetic variability corresponding to varying concentrations of manganese. The diamagnetic light blue gem shown below (left) is colored entirely by copper, and the strongly magnetic pale blue gem on the right is colored entirely by iron.
Magnetic testing cannot be used to separate medium green or dark green Paraibas from medium green or dark green "Verdelite" Tourmalines colored by iron, as both types can show a Drag response and have similar magnetic susceptibilities.
This concludes our discussion of magnetism in Tourmalines. For a comprehensive look at magnetism in the Garnet Group, which is even more diverse than Tourmaline, see our 15-page report on Garnets.
Pictured below are a light green Paraiba pear and an unusual light green copper-bearing Tourmaline oval with an odd yellow-green hue. Both gems show high magnetic susceptibility. In the odd yellowish green copper-bearing Tourmaline on the right, manganese and probably vanadium rather than copper are primarily responsible for the unusual color, and hence we do not refer to it as a true Paraiba-type Tourmaline.
© Kirk Feral 2013, All Rights Reserved. These materials may be duplicated for educational purposes only. No part of this website may be duplicated or distributed for profit, for commercial purposes, or for posting to another website without the expressed written consent of the copyright holder.
Blue Tourmaline Colored by Copper
(Mozambique, .38ct, SI <0)
The amount of copper impurities in allochromatic Paraiba Tourmalines can be very small. Researchers have found that copper oxide concentrations as low as 0.15% by weight are found in purple and violet copper-bearing Tourmalines from Mozambique, while higher concentrations are found in blue stones from Paraiba, Brazil ranging from 0.4% in light blue stones to 2.5% in dark blue stones. At these levels, copper in Paraiba Tourmalines cannot be detected with a magnet, as copper oxides are 30 times less paramagnetic than iron oxides. Copper ions (Cu2+) in copper oxide have only one unpaired electron, and consequently induce very low magnetic susceptibilities.
Paraiba Tourmaline gem colors include blue, green and violet. Copper-bearing Tourmalines with pink, red or purple colors are technically not classified as Paraiba-type Tourmalines, although gem dealers often don't adhere to that distinction. Paraiba Tourmalines typically have “neon” colors that are brighter than we find in Tourmalines without copper. This author suggests the possibility that the "neon" brightness of Paraibas might be caused by manganese (Mn2+, yellow color) brightening the color induced by copper (Cu2+, blue color).
< 4
Copper-bearing vs. Iron-bearing Blue Tourmalines
Yellowish Green Paraiba
(2.05ct., Drag, SI 373)
Blue Tourmaline Colored by Iron
Afghanistan, 7.23ct, SI 230
< Previous 1 2 3 4
Violet, Lavender and Purple: Violet, lavender and purple colors in copper-bearing Tourmaline result from a combination of pink color from manganese (Mn3+) and blue color from copper (Cu2+). Manganese in the valence state of Mn2+ can also be present in significant concentrations, as evidenced by the strong magnetic response of the violet pear shown below right. Cryptic manganese (Mn2+) ions can be responsible for strong magnetic susceptibilities without contributing color. Heat treatment is routinely applied to violet, lavender, purple, pink and red copper-bearing Tourmalines to drive out pink/red color caused by Mn3+ (Abduriyim et al., 2006), leaving only colorless Mn2+ and the desired blue color caused by copper. Heat changes the valence state of manganese from Mn3+ (pink) to Mn2+ (yellow or colorless/cryptic).
Lavender Paraiba
(2.31ct., Strong, SI 169)
Violet and lavender copper-bearing Tourmalines with a lot of blue in the body color such as the oval, pear and round gems pictured above qualify as true Paraibas. On the other hand, purple copper-bearing Tourmalines such as the one shown below are technically not considered by gemologists as Paraiba-type because the color is predominantly influenced by manganese rather than copper. The preferred terminology is copper-bearing Tourmaline. Purple copper-bearing Tourmalines contain more Mn3+ (pink color) than do violet Paraiba gems. Magnetic susceptibility measurements indicate that concentration of Mn2+ varies widely. Magnetic responses of the purple copper-bearing tourmalines we measured ranged from Weak to Strong.
Purple Copper-bearing Tourmaline
(4.76ct., Strong, SI 174)
Purple Copper-bearing Tourmaline
(2.34ct., Strong, SI 239)
The purple copper-bearing Tourmaline pear pictured below shows brown striated inclusions that are common in copper-bearing Tourmalines of various colors from Nigeria and Mozambique. It has been reported that these inclusions are iron oxides (Hematite) which stain the inner walls of growth tubes. Most probably, the thin layers of iron oxides do not contribute to detectable magnetic susceptibility.
No blue Tourmalines in this study colored by iron and iron-iron charge transfer have been found to show a Diamagnetic response as do some copper-bearing Paraibas. Also, no blue Tourmalines colored by copper have been found to show a Drag response as do iron-rich dark blue "Indicolite" Tourmalines.
Blue Paraiba Tourmaline
(Mozambique, 12.52ct, Strong, SI 273)
Pink and Red: Tourmalines that are pink or red in color and contain traces of copper are also technically not classified as Paraiba-type, although they are sometimes sold as Paraibas. The concentration of copper in these gems is necessarily very low compared to true Paraiba gems, particularly compared to blue and green gems. Any significant concentration of Cu2+ would contribute blue color, imparting a visible purple hue to pink/red gems.
When Tourmalines are pure pink or pure red, color is entirely due to manganese (Mn3+) rather than copper (Cu2+). These gems are best referred to as pink Tourmalines and "Rubellite" Tourmalines the may or may not contain some copper. As we showed on the previous page, pink Tourmalines and "Rubellite" are diamagnetic to moderately magnetic. The three gems pictured below were sold improperly as Paraiba Tourmaline or copper-bearing Tourmaline, but a spectrometer reveals no trace of copper in any of these gems.
Pink Tourmaline Sold as Paraiba
(Mozambique, 2.81ct., Inert, SI <0)
"Rubellite" Sold as Paraiba
(Nigeria, 2.77ct., Weak, SI <20)
In idiochromatic gemstone species such as blue Turquoise, copper induces weak to moderate magnetic attraction because copper within copper salts is in high concentrations (about 6% by weight). However, the strong magnetic attraction we often find in allochromatic Paraiba Tourmalines is due entirely to manganese. Among Paraiba Tourmaline gems, manganese is frequently present in much higher concentrations than copper (up to 6% manganese oxide by weight). Iron is found only as a trace element in Paraiba Tourmalines, and such an extremely low level of iron is not magnetically detectable.
Paraiba Tourmalines
Photo by Wimon Manorotkul, courtesy of palagems.com
Some red Tourmalines can have a subtle purplish hue that is due manganese (Mn3+) plus a very small amount of copper, and these can legitimately be referred to as copper-bearing Tourmalines. But gemologists still do not consider these to be Paraiba-type Tourmalines. Purplish-red copper-bearing Tourmalines from Mozambique and Nigeria are often heated to remove the red color completely, leaving a light blue color that is the result of a very low concentration of copper. The blue color that remains may appear pale and washed-out, but that doesn't stop retailers from selling these previously-red Tourmalines at high prices as "Paraiba" Tourmalines.
"Rubellite" Sold As Copper-bearing
(Mozambique, 6.68ct., Weak, SI 35)
Light Yellowish Green Cuprian Tourmaline
(1.12ct, Strong, SI 260)
Blue Paraiba Tourmaline
(Nigeria, 7.81ct, Strong, SI 234)
Blue Paraiba Tourmaline
(1.88ct., Strong, SI 221)
Fluorescence
Copper-bearing Tourmalines are the only color variety of the Elbaite species that we have found that sometimes fluoresces under longwave UV light. Fluorescence is uncommon, and most Parabias in our study do not fluoresce, as is the case with most other gem Tourmalines. But some blue and light green copper-bearing Tourmalines fluoresce a weak to moderate bluish white color due to an unidentified activator (probably a rare earth element), as pictured below right. (Note: Pink areas in the photo on the right are an artifact of UV light, not fluorescence). As we have found in our research, the only other gem Tourmaline that occasionally fluoresces under longwave UV light is Chrome Tourmaline, which belongs to the Dravite species, fluorescing red due to chromium. Some Dravite and Uvite Tourmalines can occassionally show fluorescence under shortwave UV light.
Blue Paraiba Tourmaline
2.60ct, Strong, SI 95
Daylight and LWUV Fluorescence
Light Green Paraiba
(.48ct, Strong, SI 309)
Violet Paraiba
(Mozambique, 7.11ct, Weak, SI 39)
For our study, we tested over 30 faceted copper-bearing Tourmalines (from Brazil, Nigeria and Mozambique) as well as a number of rough copper-bearing crystals. Rough crystals are mostly small and mined as worn pebbles rather than as well-formed crystals in matrix. We verified all samples as genuinely copper-bearing using a UV-Vis-NIR spectrometer to detect copper content.
Most Paraiba Tourmaline gems sold today have been heat-treated to highlight the blue or green color by eliminating any accompanying pink color. Heat treatment is also used to lighten the blue color of stones that are naturally dark blue, possibly by altering the valence of a portion of the copper ions. Apparently lighter blue colors are preferred by most buyers. Below is a photo of a dark blue Paraiba Tourmaline and light blue Paraiba Tourmaline from the Batalha mine in Paraiba, Brazil. Both these gems were cut from the same piece of rough, but only the light blue gem on the right has been subjected to heat treatment. Both gems are weakly magnetic, but heat treatment has slightly reduced the total magnetic susceptibility of the manganese (Mn2+ & Mn3+) within the gem on the right.
Unheated 0.55ct Pear and Heated 0.65ct Oval Cut from the Same Rough Stone
(Batalha Mine, Paraiba, Brazil)
Green Paraiba Tourmaline
(Mozambique, 6.11ct, Strong, SI 239)
We see a similar neon effect in lab-created synthetic blue Spinels colored blue by cobalt, with additional traces of manganese that apparently brighten the color and modify the blue color to an "aqua" or blue-green hue. This effect might also be seen in natural neon blue Apatite gems, which derive blue color from rare earth metals, and additionally contain traces of manganese.
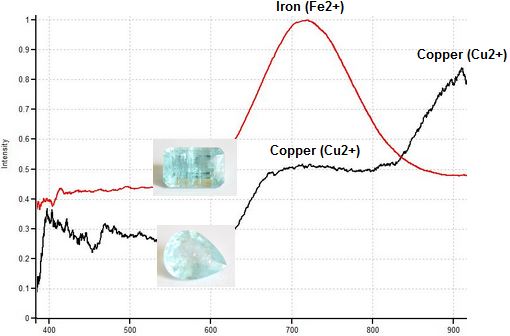
Light green cuprian Tourmalines colored by copper (Cu2+) and manganese (Mn2+) can look very similar to light green Tourmalines colored by iron (Fe2+) and iron-titanium charge transfer. Fortunately, magnetic response easily separates these two types of Tourmalines. Light green copper-bearing tourmaline is quite magnetic, often showing a Drag response due to the high concentration of manganese (Mn2+). In contrast, light green Tourmaline colored by low concentrations of iron must be floated in order for any magnetic response to be visible. All the light green copper-bearing Tourmalines tested in this study showed stronger magnetic responses and higher magnetic susceptibilities than all the light green Tourmalines colored by iron and Fe2+-Ti4+ charge transfer.
Light Green Paraiba (lft) 3.27ct., Drags, SI 330 &
Light Green Iron Tourmaline (rt), Weak, SI 26
An interesting variation of violet and lavender copper-bearing Tourmalines from Mozambique has been termed "Laurellite" by Bruce Fry (not to be confused with the lead-bearing mineral called Laurelite). This unusual Tourmaline is a Color Change Paraiba that can show a reverse Alexandrite effect by changing from violet in daylight to blue-green under yellow incandescent light (Liu, Y. & Fry, B.A.,.2006). Reverse color change from a warmer to a cooler color under warm light is a unique phenomenon.
We were able to duplicate this phenomenon under intense direct sunlight. But our examination of "Laurellites" with an incandescent flashlight and fiber optic light didn’t replicate this color change, but instead showed an unusual color diminishment phenomenon. The 2 "Laurellite" Tourmalines we tested diminish in color from violet or lavender to very pale violet or near-colorless when common incandescent light sources are applied. As far as we know, extreme color diminishment or decolorization under incandescent light is a phenomenon that hasn’t previously been described in gemology.
Lavender "Laurellite" Tourmaline, Daylight & Incandescent Light
(4.16ct., Moderate, SI 74)
Magnetism in Gemstones
An Effective Tool and Method for Gem Identification
© Kirk Feral